Tungsten Oxide: Properties, Synthesis, and Applications
Introduction
Tungsten oxide (WO³) is an inorganic compound of tungsten, well-known for its unique physical and chemical properties. It exists in several polymorphic forms, with the most common being the monoclinic phase. Tungsten oxide is utilized in a variety of applications, from catalysis and electrochromic devices to environmental and energy-related technologies. This article provides an overview of the properties, synthesis methods, and applications of tungsten oxide.
Chemical Properties
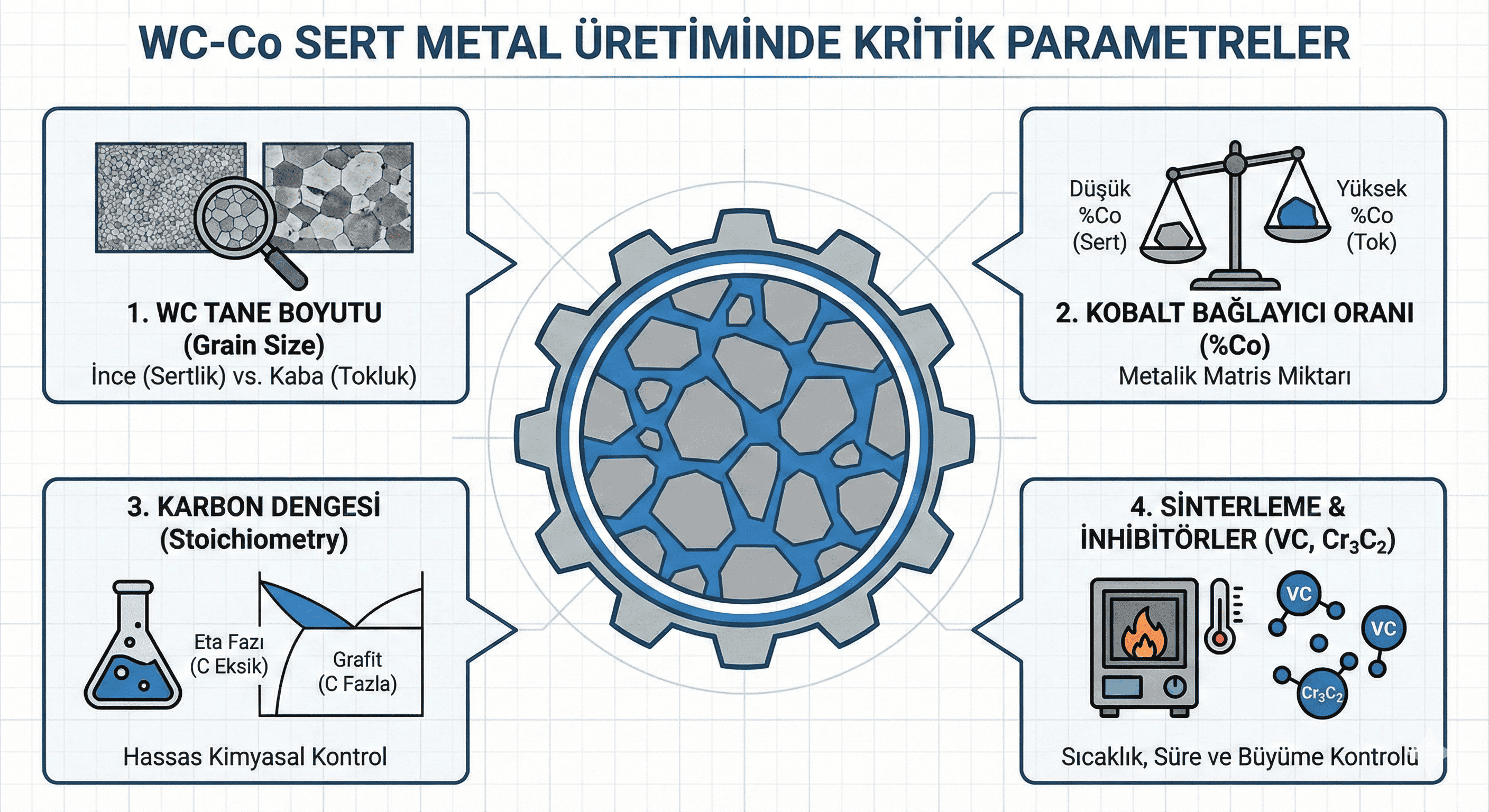
- Composition: Tungsten oxide is composed of tungsten and oxygen with a chemical formula of WO³. The most stable and common form is monoclinic tungsten oxide, although other forms include orthorhombic and tetragonal phases.
- Reactivity: WO³ is generally stable under ambient conditions but can react with strong acids and bases. It exhibits variable oxidation states, which influence its reactivity and applications, especially in catalytic processes.
- Surface Chemistry: Tungsten oxide’s surface chemistry allows for interaction with various substrates and can be modified to enhance its performance in specific applications. For instance, its surface can be functionalized to improve photocatalytic activity or electrical conductivity.
Physical Properties
- Size and Shape: Tungsten oxide can be synthesized in various forms, including nanoparticles, thin films, and bulk materials. Nanoparticles of WO³ typically range from 1 to 100 nanometers in size and can exhibit various shapes, such as spherical, rod-like, or irregular.
- Density: The bulk density of WO³ is approximately 7.16 g/cm³. The density of WO³ nanoparticles may vary depending on their size and morphology.
- Mechanical Properties: Tungsten oxide has good mechanical strength and hardness. Its mechanical properties can be enhanced by controlling particle size and shape, which is important for applications requiring durability.
- Thermal Properties: WO³ is thermally stable with a melting point of approximately 1,000°C (1,832°F). It maintains its structure and properties at elevated temperatures, making it suitable for high-temperature applications.
- Optical Properties: Tungsten oxide exhibits interesting optical properties, including variable bandgap energies depending on its phase and particle size. It is known for its photochromic and electrochromic properties, which are useful in smart windows and other optical devices.
Synthesis Methods
- Chemical Vapor Deposition (CVD): CVD is used to deposit tungsten oxide thin films from tungsten hexafluoride (WF6) or tungsten hexacarbonyl (W(CO)6) precursors. This method allows for precise control over film thickness, composition, and properties.
- Sol-Gel Method: In the sol-gel process, tungsten precursors such as tungsten alkoxides are hydrolyzed to form a gel, which is then dried and heat-treated to produce WO³. This method offers control over particle size and morphology.
- Hydrothermal Synthesis: WO³ nanoparticles can be synthesized through hydrothermal methods by reacting tungsten precursors in a high-temperature, high-pressure aqueous environment. This approach provides well-defined particle sizes and morphologies.
- Solid-State Reaction: Solid-state synthesis involves the reaction of tungsten oxides or tungsten metal with oxygen at high temperatures. This method is commonly used to produce bulk WO³ materials.
- Electrochemical Synthesis: Electrochemical methods can be used to deposit WO³ onto substrates by applying a voltage to a tungsten electrode in an electrolyte solution. This technique is useful for creating thin films and coatings.
- Ball Milling: Mechanical milling of tungsten and oxygen powders can produce WO³ nanoparticles. The high-energy milling process results in the formation of nanoscale WO³ particles through mechanical attrition.
Applications
- Catalysis: Tungsten oxide is used as a catalyst or catalyst support in various chemical reactions, including oxidation and reduction processes. Its catalytic properties are influenced by its phase and surface area.
- Electrochromic Devices: WO³ is widely used in electrochromic devices such as smart windows and mirrors. It changes color in response to an applied voltage, allowing for adjustable light transmission and privacy.
- Photocatalysis: WO³ is employed in photocatalysis for environmental applications, such as the degradation of organic pollutants and water splitting. Its ability to absorb UV light and generate reactive species makes it effective in these processes.
- Gas Sensors: Tungsten oxide is used in gas sensors to detect various gases, including nitrogen dioxide (NO²) and hydrogen. Its high sensitivity and selectivity to different gases make it suitable for environmental monitoring and safety applications.
- Energy Storage: WO³ is explored for use in energy storage devices, such as batteries and supercapacitors. Its electrochemical properties can enhance charge storage and cycling stability.
- Pigments: Tungsten oxide is used as a pigment in ceramics and glass due to its bright blue color. It provides vibrant hues and stability in high-temperature applications.
Safety and Handling
- Toxicity: Tungsten oxide is generally considered to have low toxicity. However, inhalation of fine dust or prolonged exposure should be avoided. Proper safety measures should be followed to minimize potential health risks.
- Protective Measures: When handling tungsten oxide, use appropriate personal protective equipment (PPE) such as dust masks, safety goggles, and gloves. Work in a well-ventilated area or fume hood to reduce exposure to airborne particles.
- Storage: Store tungsten oxide in airtight containers to prevent contamination and moisture absorption. Keep it in a cool, dry place to maintain stability and prevent degradation.
Conclusion
Tungsten oxide is a versatile material with a range of properties that make it suitable for diverse applications, including catalysis, electrochromic devices, photocatalysis, gas sensors, and energy storage. Understanding its synthesis methods, properties, and safety considerations is crucial for effectively utilizing tungsten oxide in advanced technologies and industrial processes.
If you have more questions or need further information, feel free to ask!