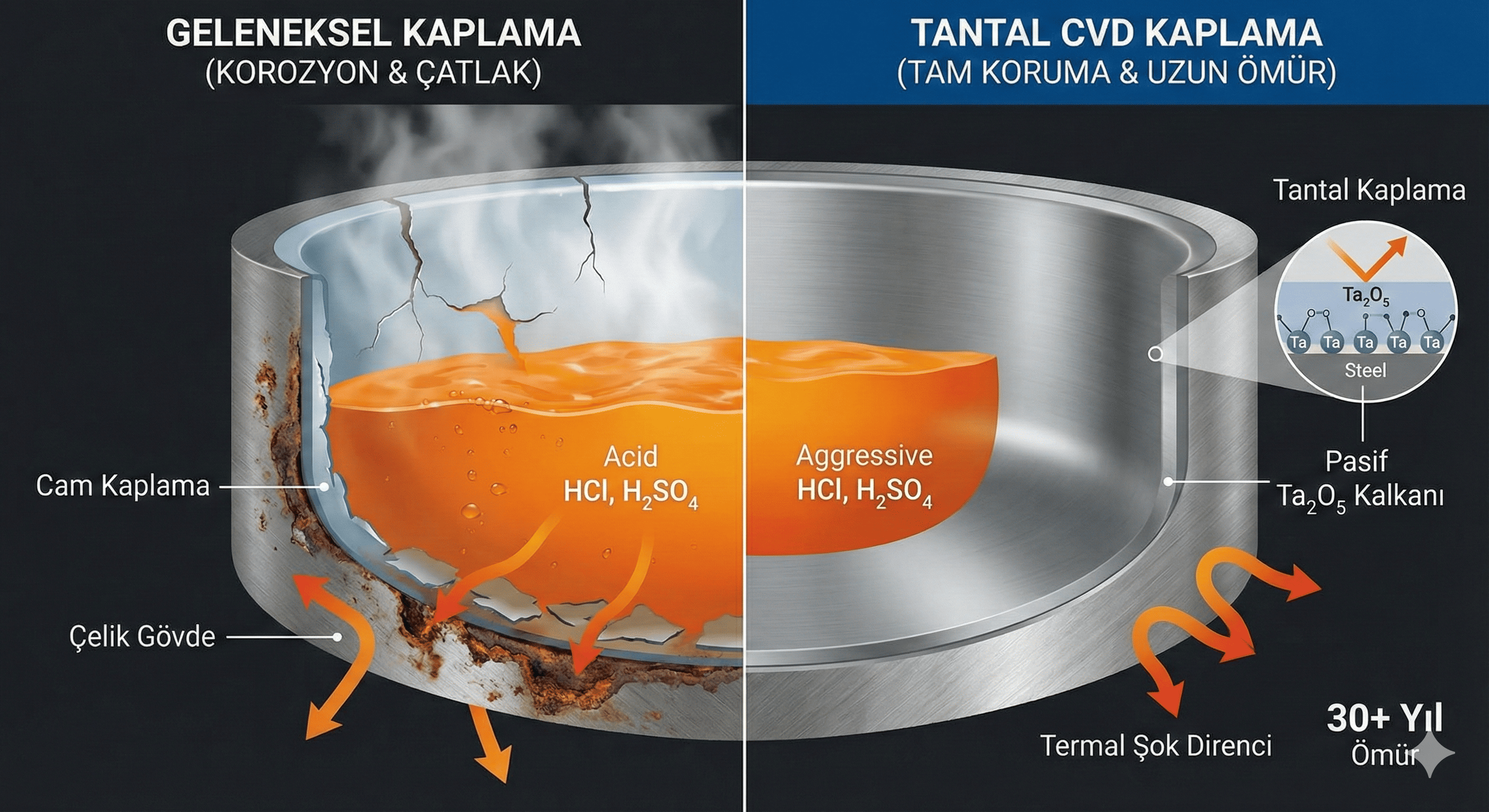
Title: Carbonyl Nickel Nanoparticles: Synthesis, Properties, and Applications
Abstract:
Carbonyl nickel nanoparticles (Ni(CO)4 NPs) represent a unique class of nickel-based nanomaterials with distinct chemical and physical properties. This article reviews the synthesis methods, characterization techniques, and diverse applications of carbonyl nickel nanoparticles, emphasizing their role in catalysis, materials science, and environmental technology.
1. Introduction:
Carbonyl nickel nanoparticles are derived from nickel carbonyl (Ni(CO)4), a volatile compound of nickel with carbon monoxide. When reduced or decomposed, Ni(CO)4 produces nickel nanoparticles with unique properties. These nanoparticles are characterized by their high surface area, reactivity, and catalytic activity, making them valuable for various technological applications.
2. Synthesis Methods:
- Thermal Decomposition: Carbonyl nickel can be decomposed thermally to produce nickel nanoparticles. The process involves heating Ni(CO)4 to high temperatures, causing it to decompose into nickel metal and carbon monoxide gas. The resulting nickel nanoparticles can be collected and processed.
- Chemical Vapor Deposition (CVD): Involves the decomposition of nickel carbonyl in a vapor phase to deposit nickel nanoparticles onto a substrate. This method allows for precise control over particle size and distribution.
- Reduction Techniques: Nickel carbonyl can be reduced using various reducing agents to produce nickel nanoparticles. This method typically involves the use of chemical reductants in solution to facilitate the reduction process.
- Solvent-based Methods: Involves the use of solvents and reducing agents to decompose nickel carbonyl in solution, resulting in the formation of nickel nanoparticles.
3. Characterization Techniques:
- Transmission Electron Microscopy (TEM): Provides high-resolution images to determine the size, shape, and internal structure of carbonyl nickel nanoparticles.
- Scanning Electron Microscopy (SEM): Offers detailed images of the surface morphology and particle size distribution of Ni NPs.
- X-ray Diffraction (XRD): Analyzes the crystal structure and phase purity of nickel nanoparticles, confirming their crystalline properties.
- Energy Dispersive X-ray Spectroscopy (EDX): Provides elemental analysis to confirm the composition of nickel nanoparticles and detect any impurities.
- Fourier Transform Infrared Spectroscopy (FTIR): Used to analyze the chemical bonding and functional groups associated with the nickel nanoparticles, especially if they are supported or modified.
4. Properties:
- Optical Properties: Carbonyl nickel nanoparticles exhibit unique optical properties due to their nanoscale size, including plasmonic effects and tunable absorption spectra.
- Catalytic Properties: Ni NPs derived from nickel carbonyl are highly active catalysts for a range of reactions, including hydrogenation, carbon monoxide oxidation, and various organic transformations. Their high surface area and reactivity make them efficient catalysts.
- Magnetic Properties: Nickel nanoparticles generally exhibit ferromagnetic behavior. The magnetic properties can be influenced by their size, shape, and the presence of surfactants or stabilizers.
- Chemical Properties: Ni NPs are prone to oxidation and must be handled carefully. They exhibit high reactivity, which is beneficial in catalytic processes but requires stabilization in practical applications.
5. Applications:
- Catalysis: Carbonyl nickel nanoparticles are used as catalysts in various chemical reactions, including hydrogenation, methanation, and Fischer-Tropsch synthesis. Their high catalytic activity and selectivity make them suitable for industrial processes.
- Materials Science: Ni NPs are incorporated into various materials to enhance their properties, such as improving the conductivity of composites or providing magnetic functionality.
- Environmental Technology: Ni NPs are used in environmental applications such as the removal of pollutants from water and air. Their catalytic activity aids in the degradation of organic contaminants and the reduction of harmful gases.
- Electronics: Due to their unique electronic properties, nickel nanoparticles are explored for use in electronic devices, including sensors and magnetic storage media.
6. Challenges and Future Directions:
While carbonyl nickel nanoparticles offer numerous advantages, challenges include their potential toxicity, stability issues, and the need for careful handling due to the volatile nature of nickel carbonyl. Future research is directed towards improving synthesis methods, enhancing the stability and performance of Ni NPs, and exploring new applications. Additionally, there is ongoing work to develop safer and more environmentally friendly methods for handling and utilizing these nanoparticles.
7. Conclusion:
Carbonyl nickel nanoparticles are a fascinating area of study with significant potential in various fields. Their unique properties, such as high catalytic activity and tunable optical characteristics, make them valuable for numerous applications. Continued advancements in synthesis, characterization, and application development will further enhance their utility and address current challenges, driving innovation in technology and materials science.
This article provides a detailed overview of Carbonyl Nickel Nanoparticles, covering their synthesis, properties, and applications. If you need more detailed information on any particular aspect or additional sections, feel free to ask